A) Only I
B) Only II
C) Only III
D) Only I and II
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkenes are terminal alkenes? 
A) I and II
B) II and III
C) I and IV
D) I and III
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest boiling point? 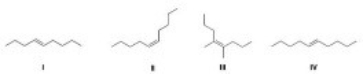
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product of the following reaction. 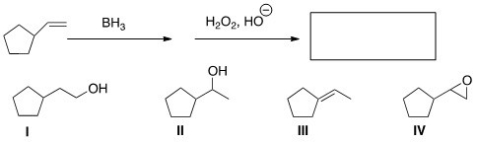
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions of alkenes takes place with anti stereochemistry only?
A) Addition of HBr
B) Addition of H2O in the presence of H2SO4
C) Addition of BH3 followed by H2O2/HO-
D) Addition of Br2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 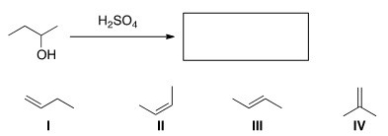
A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about alkenes is not true?
A) They are less reactive than alkanes.
B) They can exist as cis/trans isomers.
C) The bond angle around the C-C double bond is approximately 120°.
D) The carbon of the C-C double bond is sp2 hybridized.
F) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Predict the product of the following reaction. 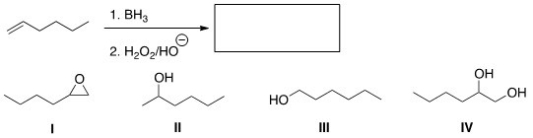
A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of reactive intermediate is formed in the reaction of an alkene with HI to give an iodoalkane?
A) Carbanion
B) Carbocation
C) Cyclic bromonium ion
D) Radical
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Rearrangements can occur in which of the following reactions?
A) Addition of Cl2 to an alkene.
B) Addition of HOBr to an alkene.
C) Addition of H2O/H3O+ to an alkene.
D) None of these.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkenes are internal alkenes? 
A) I and II
B) II and III
C) I and III
D) II and IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 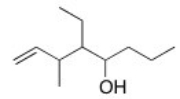
A) 4-Ethyl-3-methyl-1-octen-5-ol
B) 3-Ethyl-4-methyl-2-octen-5-ol
C) 5-Ethyl-6-methyl-7-octen-4-ol
D) 4-Ethyl-6-methyl-1-octen-5-ol
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product(s) of the following reaction. 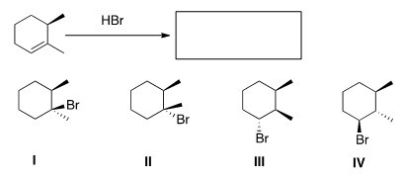
A) Only I and II
B) Only II and III
C) Only III and IV
D) Only I and IV
F) None of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to accomplish the following transformation? ![What is the best choice of reagent to accomplish the following transformation? A) H<sub>2</sub>O,Br<sub>2</sub> B) [1] BH<sub>3</sub>; [2] H<sub>2</sub>O<sub>2</sub>/HO<sup>-</sup> C) H<sub>2</sub>O,H<sub>2</sub>SO<sub>4</sub> D) [1] BH<sub>3</sub>; [2] H<sub>2</sub>O](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7dd_03ff_a9af_2f2c76dc1612_TB7662_00.jpg)
A) H2O,Br2
B) [1] BH3; [2] H2O2/HO-
C) H2O,H2SO4
D) [1] BH3; [2] H2O
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkene reacts fastest with HBr? 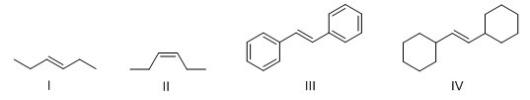
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 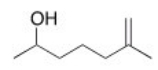
A) 5-Methyl-5-hexen-2-ol
B) 6-Methyl-6-hepten-2-ol
C) 6-Hydroxy-2-methylheptene
D) 6-Hydroxy-2-methyl-1-heptene
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reaction conditions would result in anti-Markovnikov addition to an alkene?
A) H2O/H3O+
B) HBr
C) HCl
D) [1] BH3; [2] H2O2/HO-
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the degree of unsaturation for the following molecule: 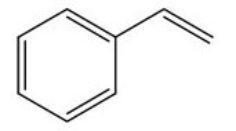
A) 2
B) 3
C) 4
D) 5
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the statements about the properties of the carbon-carbon double bond is not true?
A) There is restricted rotation around the carbon-carbon double bond.
B) Whenever the two groups on each end of a carbon-carbon double bond are the same,two diastereomers are possible.
C) Trans alkenes are generally more stable than cis alkenes.
D) The stability of the carbon-carbon double bond increases as the number of substituent groups increases.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the degree of unsaturation for the following molecule: 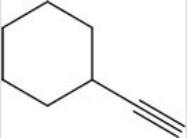
A) 2
B) 3
C) 4
D) 5
F) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 54
Related Exams