A) thermodynamics is controlling the ratio of products
B) the ratio of products is the same at all temperatures
C) thermodynamics is controlling the speed of the reaction
D) the ratio of reactants is equal to the ratio of products
E) there are both acidic and basic reactants in a reaction
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction Br2(l) → 2Br(g) ,_____.
A) ΔH is + and ΔS is +
B) ΔH is + and ΔS = 0
C) ΔH is - and ΔS is -
D) ΔH is - and ΔS is +
E) ΔH is + and ΔS is -
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
The total entropy of the universe is always increasing.This is a statement of the _____ law of thermodynamics.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At what temperatures will a reaction be spontaneous if ΔrH° = +62.4 kJ and ΔrS° = +301 J/K?
A) All temperatures below 207 K
B) All temperatures above 207 K
C) Temperatures between 179 K and 235 K
D) The reaction will be spontaneous at any temperature
E) The reaction will never be spontaneous
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction given below,ΔH0 = −1516 kJ at 25°C and ΔS0 = −432.8 J/K at 25°C.This reaction is spontaneous _____. SiH4(g) + 2 O2(g) → SiO2(s) + 2 H2O 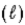
A) only below a certain temperature
B) only above a certain temperature
C) at all temperatures
D) only when entropy is zero
E) None of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a certain reversible process,q = 88.06 kJ at 29.4°C.Which of the following is the ΔS for the process?
A) 0.291 J/K
B) 291 J/K
C) 3.00 J/K
D) -0.291 J/K
E) 2590 J/K
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equilibrium constant for reaction below at 25 °C? (R = 8.314 J/K⋅mol) 2 NO(g) + O2(g) ![What is the equilibrium constant for reaction below at 25 °C? (R = 8.314 J/K⋅mol) 2 NO(g) + O<sub>2</sub>(g) 2 NO<sub>2</sub>(g) ; Δ<sub>f</sub>G° [NO(g) ] = +86.6 kJ/mol and Δ<sub>f</sub>G° [NO<sub>2</sub>(g) ] = +51.2 kJ/mol. A) 3.9 × 10<sup>-13</sup> B) 1.0 × 10-<sup>11</sup> C) 2.6 × 10<sup>12</sup> D) 1.6 × 10<sup>6</sup> E) 3.8 × 10<sup>28</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7480/11eac9a9_4b8a_9262_acc3_2f504af8e9b2_TB7480_11.jpg) 2 NO2(g) ; ΔfG° [NO(g) ] = +86.6 kJ/mol and ΔfG° [NO2(g) ] = +51.2 kJ/mol.
2 NO2(g) ; ΔfG° [NO(g) ] = +86.6 kJ/mol and ΔfG° [NO2(g) ] = +51.2 kJ/mol.
A) 3.9 × 10-13
B) 1.0 × 10-11
C) 2.6 × 1012
D) 1.6 × 106
E) 3.8 × 1028
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
While diluting concentrated sulfuric acid with water,the temperature of the solution increases rapidly.What are the signs of ΔrH,ΔrS,and ΔrG for the process?
A) ΔrH < 0,ΔrS > 0,and ΔrG < 0
B) ΔrH < 0,ΔrS < 0,and ΔrG < 0
C) ΔrH < 0,ΔrS > 0,and ΔrG > 0
D) ΔrH > 0,ΔrS > 0,and ΔrG < 0
E) ΔrH > 0,ΔrS < 0,and ΔrG > 0
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning entropy is not correct?
A) The entropy of a system increases as the number of available microstates increases.
B) The entropy of a system is proportional to the natural log of the number of microstates.
C) In a spontaneous process,ΔS(universe) indicates the extent to which energy is dispersed.
D) The dispersal of matter,such as the spontaneous expansion of a gas,cannot be explained by an increase in entropy.
E) Entropy is a measure of the extent of energy dispersal.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of a reaction that is product-favored?
A) Q < K and ![]() rG < 0
rG < 0
B) Q < K and ![]() rG > 0
rG > 0
C) Q = K and ![]() rG = 0
rG = 0
D) Q > K and ![]() rG < 0
rG < 0
E) Q > K and ![]() rG > 0
rG > 0
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following relationships is not true?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the given data,determine ΔrG° at 500.0 K for the reaction below. Ba(s) + H2O(g) → BaO(s) + H2(g) Substance ΔfH°(kJ/mol-rxn) at 298 K S° (J/K·mol-rxn) at 298 K Ba(s) 0 62) 8 H2O(g) -241.8 188) 7 BaO(s) -592 70) 4 H2(g) 0 130) 6
A) 325.0 kJ/mol-rxn
B) -325.0 kJ/mol-rxn
C) 335.2 kJ/mol-rxn
D) -335.2 kJ/mol-rxn
E) -375.5 kJ/mol-rxn
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest standard entropy per mole at 298 K?
A) H2O(l)
B) CaCO3(s)
C) CO(g)
D) SiO2(s)
E) CH3OH(l)
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following changes lead to a decrease in entropy?
A) Sugar dissolving in coffee
B) Diffusion of perfume throughout a room
C) Evaporation of gasoline
D) The sublimation (vaporization) of dry ice (solid carbon dioxide)
E) Condensation of steam on glass
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the third law of thermodynamics as defined by Ludwig Boltzmann?
A) A perfect crystal at 0 K has zero entropy.
B) In a spontaneous process,the entropy of the universe increases.
C) The total entropy of the universe is always increasing.
D) The total mass of the universe is constant.
E) Mass and energy are conserved in all chemical reactions.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following substances is the standard free energy of formation not equal to zero at 298 K?
A) Xe(g)
B) Sn(s)
C) N2(g)
D) Mg(g)
E) Mn(s)
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a chemical reaction is exothermic but not spontaneous,which of the following must be true?
A) ΔrG > 0,ΔrS > 0,and ΔrH > 0
B) ΔrG < 0,ΔrS > 0,and ΔrH > 0
C) ΔrG > 0,ΔrS < 0,and ΔrH > 0
D) ΔrG < 0,ΔrS < 0,and ΔrH < 0
E) ΔrG > 0,ΔrS < 0,and ΔrH < 0
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a reaction,ΔrH° = -208.8 kJ and ΔrS° = -308.2 J/K.At what temperature will ΔrG° = 0.00 kJ?
A) 0.68 K
B) 677.5 K
C) 1476 K
D) 6435 K
E) 0.85 K
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate ΔrG° for the reaction below at 425 °C, 2 HI(g) 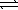 H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
A) 6.12 × 103 kJ/mol-rxn
B) 1.05 × 104 kJ/mol-rxn
C) 1.42 × 104 kJ/mol-rxn
D) 2.33 × 104 kJ/mol-rxn
E) 3.34 × 105 kJ/mol-rxn
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
ΔG° = 0 for a reaction indicates that _____.
A) the reaction favors formation of products
B) the reaction is at equilibrium
C) the reaction is nonspontaneous
D) the reaction is spontaneous
E) the reaction cannot reach equilibrium
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 66
Related Exams