A) 1 > 2 > 3
B) 2 > 3 > 1
C) 3 > 2 > 1
D) 2 > 1 > 3
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to achieve the following reaction? 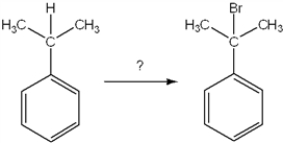
A) Br2, CCl4
B) HBr, H2O
C) Br2, light
D) NaBr
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is aromatic?
A) ethane
B) cyclobutadiene
C) benzene
D) cyclooctatetraene
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation of why the cyclopentadienyl anion (below) is aromatic. Your answer should identify the hybridization of each carbon atom and account for which electrons are in the pi system. 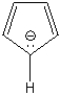
Correct Answer

verified
The all five carbons are sp2 hybridized. ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Assuming that the sulfur atom is sp2-hybridized, there are _____ π-electrons in the sulfathiazole ring.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true about [18]annulene? ![Which of the following is not true about [18]annulene? A) [18]annulene is planar B) [18]annulene is aromatic C) [18]annulene gives one peak in the <sup>1</sup>H NMR spectrum D) [18]annulene has 18 pi bonds](https://d2lvgg3v3hfg70.cloudfront.net/TB7078/11ead7c8_9d41_8819_84b0_2f725abe67cc_TB7078_00.jpg)
A) [18]annulene is planar
B) [18]annulene is aromatic
C) [18]annulene gives one peak in the 1H NMR spectrum
D) [18]annulene has 18 pi bonds
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 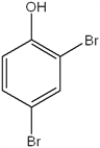
A) 2,4-dibromotoluene
B) 2,4-dibromophenol
C) 2,4-dibromohydroxybenzene
D) 4,6-dibromophenol
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to achieve the following reaction? 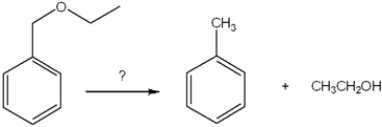
A) H2SO4
B) NaOH, H2O
C) K2Cr2O7, H2SO4
D) H2/Pd
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic) ? 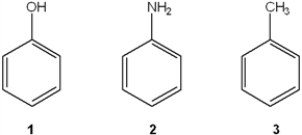
A) 1 > 2 > 3
B) 2 > 1 > 3
C) 3 > 2 > 1
D) 1 > 3 > 2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many p orbital electrons are present in cyclopentadienyl anion?
A) 4
B) 6
C) 7
D) 8
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct assignment of the names of the following heterocycles? 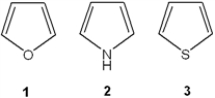
A) 1 = pyrrole; 2 = thiophene; 3 = pyridine
B) 1 = thiophene; 2 = furan; 3 = pyrrole
C) 1 = furan; 2 = pyrrole; 3 = thiophene
D) 1 = furan; 2 = thiophene; 3 = pyrrole
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 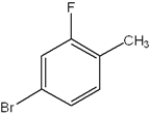
A) 1-bromo-2-fluoro-4-toluene
B) 4-bromo-2-fluorotoluene
C) 2-bromo-4-fluorophenol
D) 4-bromo-2-fluoroxylene
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the oxygen atom of furan?
A) s
B) sp
C) sp2
D) sp3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Why are nitrophenols more acidic than phenols?
Correct Answer

verified
Nitro phenols are more acidic than pheno...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic) ? 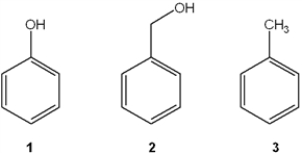
A) 1 > 2 > 3
B) 2 > 1 > 3
C) 3 > 2 > 1
D) 1 > 3 > 2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the relative positions of the substituents in the following structure? 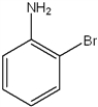
A) anti
B) meta
C) ortho
D) para
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the relative positions of the substituents in the following structure? 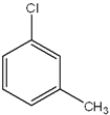
A) anti
B) meta
C) ortho
D) para
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following structure. 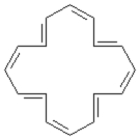 -This structure contains ____ π electrons.
-This structure contains ____ π electrons.
Correct Answer

verified
Correct Answer
verified
True/False
Phenols are stronger acids than alcohols because of the resonance stabilization of alkoxide ions.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 77
Related Exams