B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds gives rise to an absorbance at approximately 2700-3300 cm−1 in the infrared spectrum?
A) C≡C
B) C=O
C) C=C
D) C−H
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the energy of a photon moving with a frequency of 10.0 × 1010 s−1? (Planck's constant = 6.626 × 10−34 J s)
A) 6.626 × 10−23 J
B) 6.626 × 10−22 J
C) 66.26 × 10−23 J
D) 66.26 × 10−25 J
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds gives the weakest peak in the infrared spectrum for a stretching vibration? 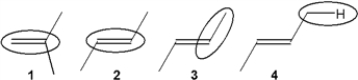
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds gives an infrared spectrum with peaks at 3300 cm−1 (sharp peak) and 2150 cm−1 (sharp peak) ? 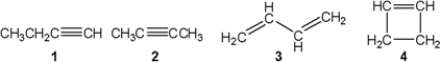
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds gives an infrared spectrum with peaks at 1000-1250 cm−1 (strong) , but does not have peaks at 3200-3650 or 1630-1820 cm−1 (sharp, weak peak) ? 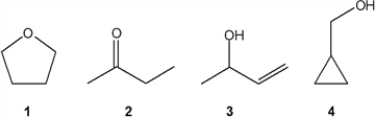
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Cyclohexene and 2-hexyne both have the molecular formula C6H10. Any of the following regions of an IR spectrum could be used to distinguish these two compounds. 3020-3100 cm−1 2100-2260 cm−1 1650-1670 cm−1
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following relationships is not valid as applied to infrared spectroscopy?
A) wavenumber = 1/λ (in cm)
B) ΔE = h ν
C) ΔE = λ ν
D) ν = 4.12 √(K/μ)
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds gives a carbonyl bond absorption at the lowest frequency? 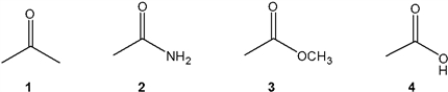
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
An IR spectrum of an unknown organic molecule having the formula which contains carbon, hydrogen, and oxygen reveals a strong absorption at 1715-1725 cm-1. This molecule is most likely an ether.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds gives a carbonyl bond absorption at the highest frequency? 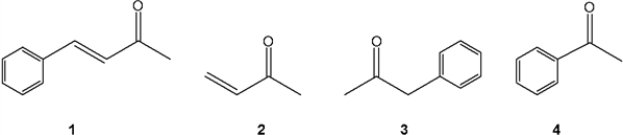
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the frequency of a radiation with a wavelength of 3.0 × 10−5 m? (Speed of light = 3.0 × 108 m/s)
A) 1 × 1013 Hz
B) 2 × 1013 Hz
C) 1.1 × 1013 Hz
D) 1.2 × 1013 Hz
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Label three peaks in the following spectrum with the bond that gives rise to them, and identify the functional group that is present in the compound (the compound has a formula of C5H10O2)?
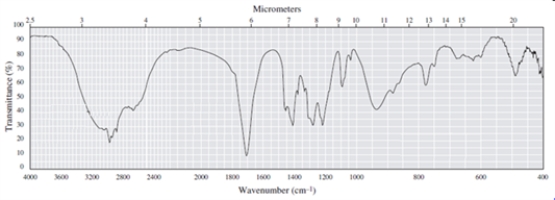
Correct Answer

verified
O-H: 3000-3600 cm-1
C-...View Answer
Show Answer
Correct Answer
verified
C-...
View Answer
Multiple Choice
Which of the following regions in the electromagnetic spectrum corresponds to the radiation with the highest energy?
A) radio waves
B) ultraviolet
C) infrared
D) visible
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 74 of 74
Related Exams