A) TΔS° = 0
B) −TΔS° > ΔH°
C) TΔS° < 0°
D) ΔG° = 0
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of trimethylamine, (CH3) 3N?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of curved arrows accounts for the deprotonation of an acid (A-H) by a base (:B) ? 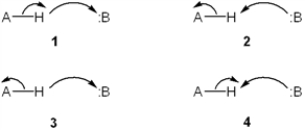
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the acid dissociate constant, Ka,for the following equilibrium.
Correct Answer

verified
\(K_{\mathrm{a}}=\left[\mathrm{H}_{2} \mathrm{O}\right] K_{\mathrm{eq}}=\frac{\left[\mathrm{A}^{-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{[\mathrm{HA}]}\)
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values? CH3CH3 > CH2=CH2 > HCCH ethane ethene ethyne (ethylene) (acetylene) 51 44 25
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3OCH3
B) CH3CH2OH
C) CH3CHO
D) CH3CO2H
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values? CH3OH > CH3NH2 > CH3CH3 16 38 51
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is the strongest base?
A) iodide anion, I−
B) fluoride anion, F−
C) bromide anion, Br−
D) chloride anion, Cl−
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CF3OH
B) CF3CH2 CH2OH
C) CF3CH2 CH2 CH2OH
D) CF3CH2 CH2 CH2CH2OH
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
An acid with a low pKa is a strong acid and has a weak conjugate base.
B) False
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethene in the acid-base reaction shown? 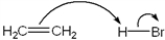
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of the equilibrium constant, Keq, for the following reaction? 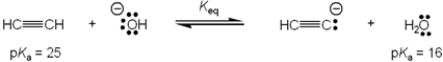
A) 109
B) 10−9
C) 9
D) . 1/9
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the lowest pKa?
A) H2O
B) HBr
C) NH3
D) CH4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
In the following list, there are two organic Lewis bases.

B) False
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is present in the highest concentration upon dissolution of H2SO4 in water?
A) H2SO4
B) H+
C) H3O+
D) HO−
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery.  Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3CH2CH2COOH
B) CH3CH2CHClCOOH
C) CH3CHClCH2COOH
D) ClCH2CH2CH2COOH
F) All of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of boron tribromide, BBr3?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 94
Related Exams