A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is easiest to deprotonate?
A) CH4
B) CH3CH3
C) CH2=CH2
D) HC≡CH
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 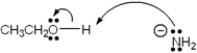
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has a pKa value of approximately 16?
A) HBr
B) CH3COOH
C) CH3CH2OH
D) HC≡CH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3OH
B) CH3CHO
C) CH3COCH3
D) CH3COOH
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3NH2
B) CH3PH2
C) CH3OH
D) CH3SH
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Gaseous HCl when dissolved in toluene is a _____.
A) weak acid
B) strong acid
C) proton acceptor
D) hard acid
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values? CH3CH2OH > CH3COOH 15.9 4.76
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following energy diagrams best represents the changes in energy during addition of HBr to an alkene? 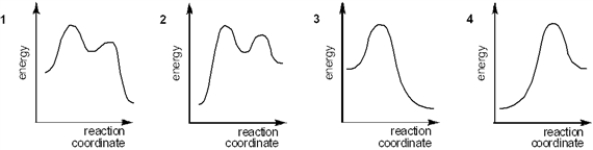
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following compounds in the order of their decreasing basicity: Guanidine, Urea, Amidine
A) Guanidine > Amidine > Urea
B) Guanidine > Urea > Amidine
C) Urea > Guanidine > Amidine
D) Amidine > Guanidine > Urea
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
In the following list, there are four Lewis acids, CH3OH, HCl, BCl3 and FeBr3. 
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the types of reactions with the most appropriate relationships. -ΔGproduct > ΔGreactant
A) Exergonic Reaction
B) Endergonic Reaction
C) Exothermic Reaction
D) Endothermic Reaction
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3CH3
B) CH3NH2
C) CH3OH
D) CH3F
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest base?
A) NaOH
B) NaCO3
C) H2O
D) CH3OH
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Explain the highly basic nature of guanidine.
Correct Answer

verified
Guanidine is highly basic in nature beca...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which species is the conjugate acid in the following acid-base reaction? 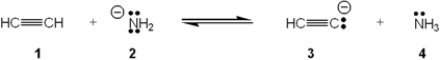
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the strongest base?
A) CH3COO−
B) HO−
C) NH2−
D) Cl−
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethyne in the acid-base reaction shown? 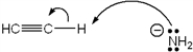
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the equilibrium constant, Keq, for the following equilibrium.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 94
Related Exams