A) HCl
B) HI
C) HF
D) HBr
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Why are phenols more acidic than alcohols?
Correct Answer

verified
Phenols are more acidic than a...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 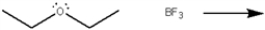
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom in the following structure is preferentially protonated by a strong acid? 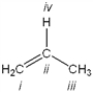
A) i
B) ii
C) iii
D) iv
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A) CH3COOH
B) AlCl3
C) H2O
D) CH3OH
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest pKa?
A) SiH4
B) H2S
C) PH3
D) HCl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the equation that relates the equilibrium constant, Keq, to the acid dissociation constant, Ka, for the following equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the types of reactions with the most appropriate relationships. -ΔHproduct > ΔHreactant
A) Exergonic Reaction
B) Endergonic Reaction
C) Exothermic Reaction
D) Endothermic Reaction
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct order of decreasing basicity (stronger base > weaker base) ?
A) NH3 > MeNH2 > H2O > HF
B) MeNH2 > NH3 > MeOH > CH4
C) NH3 > Me3N > H2O > MeOH
D) CH3COONa > NaOH > NaOMe > NaNMe2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
A) OH−
B) H3O+
C) CH3COOH
D) CH3COOH+
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is correct?
A) ΔG° = ΔH° − TΔS°
B) ΔH° = ΔG° − TΔS°
C) ΔG° = ΔH° − ΔS°
D) ΔG° = ΔH° − ΔS°/T
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 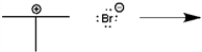
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sets of curved arrows accounts for the protonation of propene with HI? 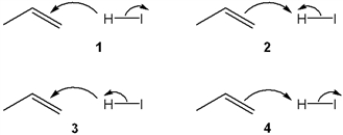
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The following is generic depiction of a reaction using the curve arrow formalism. 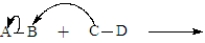 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 81 - 94 of 94
Related Exams