A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What two families of compounds react in a step growth polymerization to give a polyester?
A) alcohol/carboxylic acid
B) carboxylic acid/amine
C) alcohol/amine
D) alcohol/ether
E) carboxylic acid/ether
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures would represent the repeating unit of an alternating styrene-acrylonitrile copolymer? 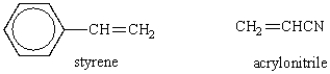
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these represent the copolymer unit.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isoprene unit is common in many synthetic and natural polymers.Isoprene is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The repeating unit of polypropylene is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of polymer continues to crosslink on heating to produce a hard, infusible material?
A) thermosetting
B) vulcanized
C) thermoplastic
D) linear
E) copolymer
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following arrangements indicate a crosslinked homopolymer?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) both B and C
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following polymers can be classified as a thermoplastic?
A) bakelite
B) polyurethane
C) urea-formaldehyde
D) polystyrene
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nylon 6 is a polymer of caprolactam.What is the structure of caprolactam?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following arrangements would indicate a graft copolymer?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The repeating monomer unit in styrofoam cups is:
A) ethylene
B) vinyl chloride
C) styrene
D) tetrafluoroethylene
E) propene
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An example of a polyether that can be prepared from the epoxide ethylene oxide is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A polymer has the repeating unit drawn below: 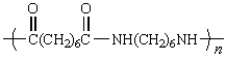 From what two monomers is this polymer made?
From what two monomers is this polymer made?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vulcanization is a process to strengthen natural rubber by crosslinking polymer chains with what element?
A) sodium
B) titanium
C) sulfur
D) aluminum
E) phosphorus
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The process by which rubber is cross-linked is called
A) polymerization.
B) epimerization.
C) saponification.
D) vulcanization.
E) solidification.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound that can be used to crosslink poly(vinyl alcohol) by ester linkages is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which monomer can be used to prepare the following polymer? 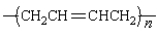
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Kel-F is the trade name for a copolymer used for rocket motors.Which monomer units will produce this copolymer? 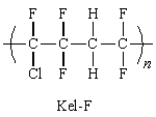
A) CH2=CF2 and Cl2C=CF2
B) CH2=CH2 and CF2=CCl2
C) ClFC=CF2 and CH2=CF2
D) CF2=CF2 and CH2=CHF
E) CHCl=CH2 and CH2=CF2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The preparation of which step-growth polymer does not involve elimination of a small molecule during the course of its formation?
A) urea-formaldehyde
B) polyamides
C) bakelite
D) polyurethanes
E) polyesters
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines the density of a polyurethane foam?
A) the amount of ammonia gas released
B) the amount of carbon dioxide released
C) the amount of oxygen absorbed
D) the solubility of the reactants
E) the amount of nitrogen absorbed
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 49
Related Exams