B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
If water accumulates between two sheets of glass, it is very difficult to separate them. Which of the following explanations best explains this situation?
A) The cohesive property of water holds the two sheets of glass together.
B) The adhesive property of water holds the sheets of glass together.
C) The nonpolar covalent bonds in the class are attracted to the polar covalent bonds of the water, holding the two sheets of glass together.
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements accurately describes lipids?
A) Lipids are the building blocks of carbohydrates.
B) Lipids serve as buffers.
C) Lipids are an important component of plasma membranes.
D) Lipids tend to be water soluble.
E) Lipids tend to be polarized.
G) B) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the building block molecule of a protein?
A) Amino acid
B) Nucleic acid
C) Monosaccharide
D) Glycerol
E) Fatty acid
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown molecule was detected in a tissue sample. The molecule is composed of carbon, hydrogen, and oxygen; appears to be a branching polymer; and is hydrophilic. This molecule is most likely a ________.
A) carbohydrate
B) lipid
C) protein
D) nucleic acid
F) None of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
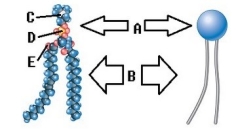 -Phospholipids are important components of the plasma membrane. What does "A" represent on the diagram?
-Phospholipids are important components of the plasma membrane. What does "A" represent on the diagram?
A) Phosphorus
B) Oxygen
C) Nitrogen
D) Polar (hydrophilic) region
E) Nonpolar (hydrophobic) region
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To which of the following organic groups does hemoglobin belong?
A) Carbohydrate
B) Protein
C) Lipid
D) Nucleic acid
E) Vitamin
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sucrose is formed when the simple sugars fructose and glucose are covalently bonded. This reaction releases water. What type of reaction is this?
A) Catabolic
B) Hydrolysis
C) Dehydration
D) Monomeric
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hyperventilation causes the loss of large amounts of CO2 from the body, decreasing the amount of H+ in solution. As a result,
A) the pH of body fluids will rise.
B) the pH of body fluids will fall.
C) the pH of body fluids will become neutral.
D) the pH of body fluids will not be affected.
E) None of these choices are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An example of a fat-soluble vitamin is
A) vitamin C.
B) vitamin D.
C) vitamin B.
D) vitamin F.
E) vitamin H.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
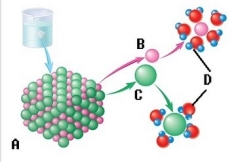 -The sodium chloride molecule breaks apart in water. What does "D" represent?
-The sodium chloride molecule breaks apart in water. What does "D" represent?
A) Chloride ion
B) Dissociation
C) Water molecule
D) Sodium ion
E) Salt crystal
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hydrogen and oxygen atoms in a molecule of water are held together by ________ bonds.
A) ionic
B) peptide
C) savings
D) polar covalent
E) nonpolar covalent
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An anion is
A) a combination of atoms held together by chemical bonds.
B) a positively charged ion.
C) a negatively charged ion.
D) a molecule that conducts electricity when placed in solution.
E) an alteration in the three-dimensional structure of a protein.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An individual hydrogen bond in a sample of water would be described as
A) strong and intramolecular.
B) strong and intermolecular.
C) weak and intramolecular.
D) weak and intermolecular.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -The sodium chloride molecule breaks apart in water. What does "A" represent?
-The sodium chloride molecule breaks apart in water. What does "A" represent?
A) Chloride ion
B) Dissociation
C) Water molecule
D) Sodium ion
E) Salt crystal
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the material that would NOT be considered an important inorganic substances in our bodies.
A) Glucose
B) Oxygen
C) Calcium
D) Iron
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phospholipids have a hydrophilic end which is
A) polar and not water soluble.
B) polar and water soluble.
C) nonpolar and not water soluble.
D) nonpolar and water soluble.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a reversible reaction, when the rate of product formation is equal to the rate of reactant formation, the reaction is
A) stopped.
B) at equilibrium.
C) in danger of exploding.
D) a net decomposition reaction.
E) a net synthesis reaction.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium chloride is considered a/an ________.
A) molecule
B) compound
C) Both molecule and compound are correct.
D) element
E) ion
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nucleotides
A) are part of DNA molecules but not RNA molecules.
B) hold the nucleus together.
C) are the building blocks of nucleic acids.
D) are proteins that function as enzymes.
E) have nothing to do with the genetic information in the nucleus.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 207
Related Exams