A) Meisenheimer complex
B) benzyne
C) cyclohexadienyl cation
D) benzyl radical
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following undergoes the most rapid nitration upon treatment with HNO3/H2SO4?
A) toluene
B) benzene
C) bromobenzene
D) nitrobenzene
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following sequence of reactions (if a reaction is likely to give you a mixture of ortho and para disubstituted compounds you should assume that they can be separated; continue the synthesis with either one)? 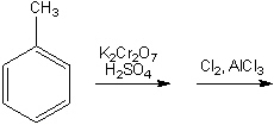
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following sequence of reactions? 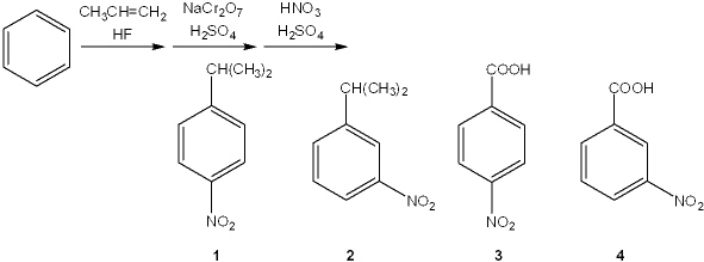
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true about Meissenheimer complexes?
A) They are resonance-stabilized anions
B) They are formed upon addition of a nucleophile to aryl halides
C) They are aromatic
D) They are intermediates in nucleophilic aromatic substitution reaction which take place by an addition-elimination mechanism
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following chlorobenzenes produces a single isomer upon treatment with NaNH2? 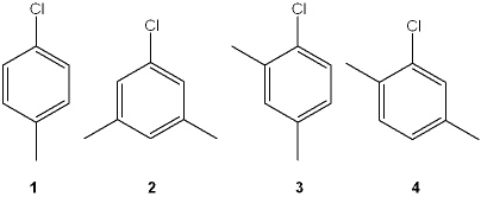
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sets of substituents are all deactivating groups in electrophilic aromatic substitution reactions?
A) Cl, OH, CH2CH3
B) CH3, Br, COCH3
C) CH3, NH2, OH
D) COCH3, NO2, Br
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which mechanism accounts for the reaction of chlorobenzene with acetyl chloride and AlCl3 to form 4-chloroacetophenone?
A) Bimolecular nucleophilic substitution (SN2)
B) Nucleophilic aromatic substitution by elimination-addition
C) Nucleophilic aromatic substitution by addition-elimination
D) Electrophilic aromatic substitution
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the reactive intermediate formed in the electrophilic bromination of toluene with Br2, FeBr3? 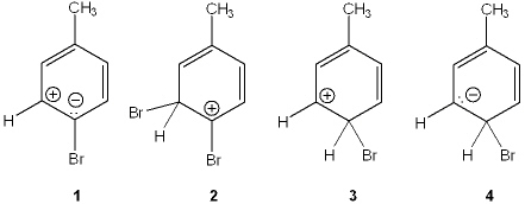
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sets of substituents are all meta directing in electrophilic aromatic substitution reactions?
A) CH3, OH, Br
B) Cl, NH2, CN
C) COCH3, NO2, COOH
D) NH2, COCH3, OH
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 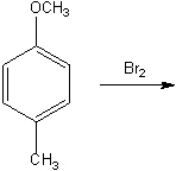
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cannot be prepared from benzene in two synthetic steps? 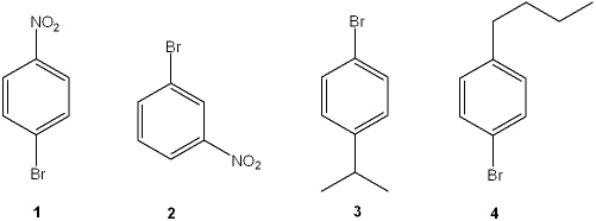
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which mechanism accounts for the reaction of benzyl chloride with sodium cyanide to form cyanomethylbenzene (phenylacetonitrile, PhCH2CN) ?
A) Bimolecular nucleophilic substitution (SN2)
B) Nucleophilic aromatic substitution by elimination-addition
C) Nucleophilic aromatic substitution by addition-elimination
D) Electrophilic aromatic substitution
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following undergoes the most rapid acylation upon treatment with acetyl chloride and AlCl3?
A) benzene
B) toluene
C) chlorobenzene
D) 1,4-dichlorobenzene
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does anisole undergo bromination with Br2 in the absence of FeBr3 whereas bromination of benzene requires the presence of FeBr3?
A) anisole is a better nucleophile than benzene
B) the methoxy group of anisole is an inductive electron withdrawing substituent
C) Br2 is a good nucleophile
D) the reaction is accelerated by light
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does nitrobenzene fail to undergo Fieldel-Crafts alkylation with tert-butyl chloride and AlCl3?
A) nitrobenzene reacts with AlCl3
B) nitrobenzene is a poor nucleophile
C) the nitro group is a strong electron donating group
D) the tert-butyl cation is a poor electrophile
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true about benzynes?
A) They are planar
B) They are formed upon dehydrohalogenation of aryl halides
C) They are aromatic
D) They are intermediates in nucleophilic aromatic substitution reaction which take place by an addition-elimination mechanism
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
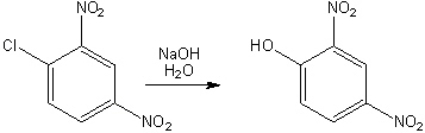
Provide the structure (a single resonance contributor) of the key intermediate in the following reaction
. 
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction (assume that mixtures of ortho and para disubstituted compounds can be separated; continue the synthesis with either one)? 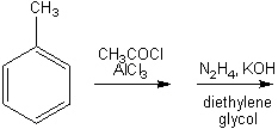
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 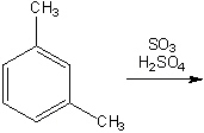
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 83
Related Exams