A) (E) 6,6-dimethyl-1-hexene
B) (E) 5,5-dimethyl-1-heptene
C) (E) 6,6-dimethyl-1-heptene
D) (E) 6,6-dimethyl-2-heptene
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alcohols can be prepared from a Grignard reagent and ethylene oxide? 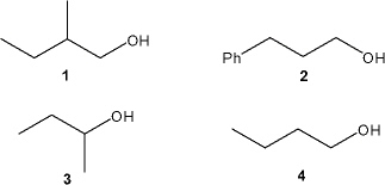
A) only 1 and 2
B) only 1 and 4
C) only 1, 2 and 3
D) only 2 and 4
F) A) and B)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following sequence of reactions? 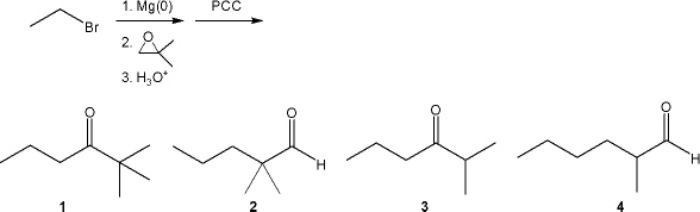
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following sequence of reactions?
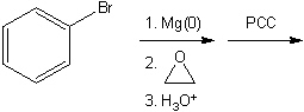
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best choice of solvent for the formation of phenyllithium by the reaction of bromobenzene with lithium?
A) water
B) ethanol
C) tetrahydrofuran
D) acetic acid
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 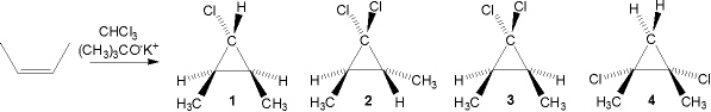
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is he change in oxidation state of the metal when magnesium reacts with an alkyl bromide to give an alkylmagnesium bromide (Grignard reagent) ?
A) 0 +1
B) 0 +2
C) 0 -1
D) 0 -2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best choice of solvent for the formation of phenylmagnesium bromide by the reaction of bromobenzene with magnesium?
A) water
B) methanol
C) diethyl ether
D) acetic acid
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 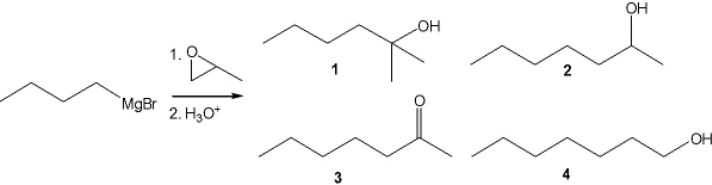
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following sequence of reactions? 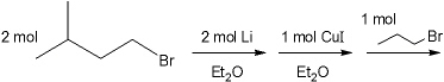
A) (Z) -6-methyl-2-heptene
B) (E) -4-methyl-2-pentene
C) 2-methylheptane
D) 3-methylheptane
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 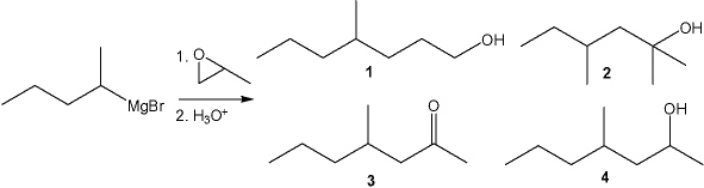
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination(s) of alkyl bromide and epoxide can be used to prepare the following product by addition of the Grignard reagent derived from the alkyl bromide to the epoxide? 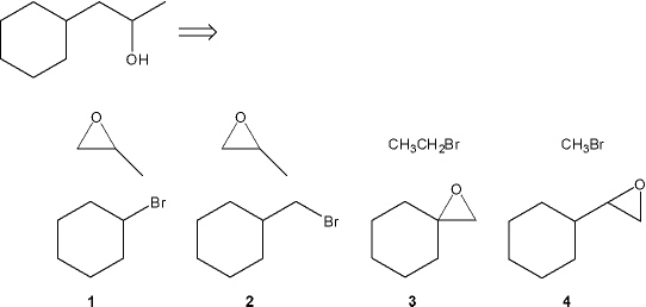
A) only 1
B) only 2
C) only 1 and 3
D) only 2 and 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest base?
A) CH3CH2MgBr
B) CH3CH2OH
C) CH3CH2OMgBr
D) CH3CH3
F) All of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 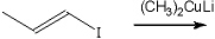
A) (E) 2-iodo-2-butene
B) 1-iodo-2-methylpropene
C) methylcyclopropane
D) (E) 2-butene
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 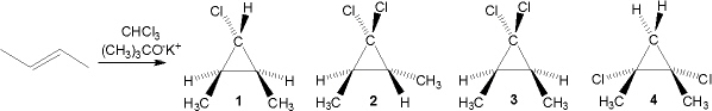
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction?
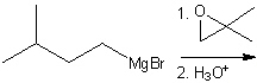
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following sequence of reactions? 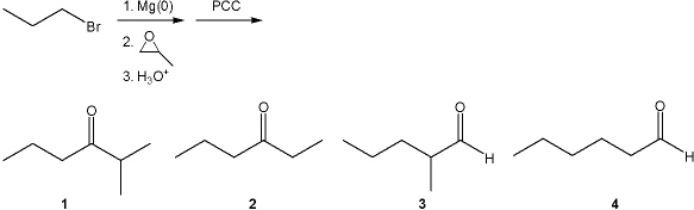
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 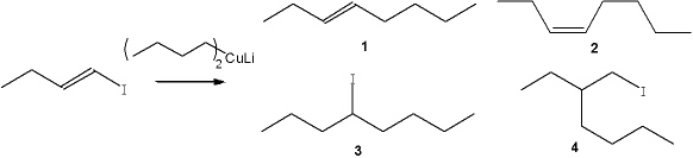
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true regarding the carbon atom of the methylene carbene, H2C: ?
A) it is sp2 hybridized
B) it has a complete octet of valence electrons
C) it has a vacant 2p atomic orbital
D) it has a lone pair of electrons in an sp2 molecular orbital
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination(s) of alkyl bromide and epoxide can be used to prepare the following product by addition of the Grignard reagent derived from the alkyl bromide to the epoxide? 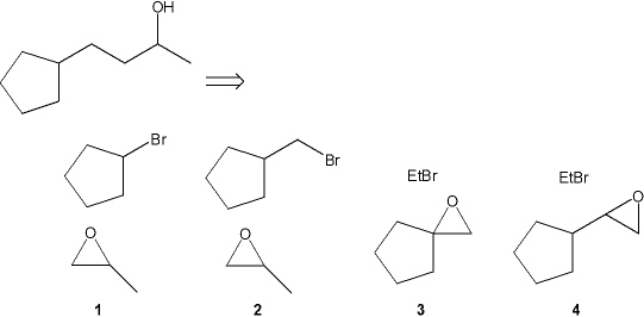
A) only 1
B) only 2
C) only 1 and 3
D) only 2 and 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 45
Related Exams