Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole? 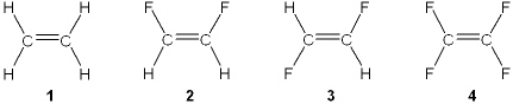
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole?
A) H2O
B) CO2
C) HCºCH
D) Cl2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Convert the following structure into a bond-line drawing.
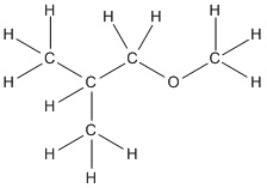
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C-H s bonding molecular orbital in ethene, H2C=CH2 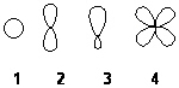
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can the shell with a principal quantum number of 1 hold?
A) 1
B) 2
C) 4
D) 8
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following resonance structures makes the largest contribution to the structure of [H2CCHO] -? ![Which of the following resonance structures makes the largest contribution to the structure of [H<sub>2</sub>CCHO]<sup> -</sup>? A) 1 B) 2 C) 3 D) 4](https://d2lvgg3v3hfg70.cloudfront.net/TB7077/11eb135c_c799_b370_8021_4b082ca9a792_TB7077_00.jpg)
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon s molecular bonding orbital of ethyne, HCºCH?
A) C2p + C2p
B) C2sp + C2sp
C) C2sp2 + C2sp2
D) C2sp3 + C2sp3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethyne (acetylene, HCºCH). Label each bond (e.g., C-H s bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the molecular formula of Ritalin, shown below?
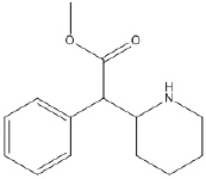
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is the most polar?
A) O-H
B) C-H
C) C-C
D) H-H
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of a 2p atomic orbital of carbon? 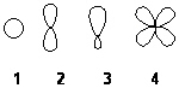
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds has the smallest dipole moment?
A) C-N
B) C-O
C) C-F
D) O-H
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a carboxylic ester?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2COOCH3
D) CH3CH2COCH3
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethene, H2C=CH2?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can the shell with a principal quantum number of 2 hold?
A) 1
B) 2
C) 4
D) 8
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a nitrogen atom (nitrogen: atomic number 7) ?
A) 1s22s12p4
B) 1s22s22p3
C) 1s12s12p5
D) 1s22s22p2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is trigonal planar?
A) boron trifluoride, BF3
B) methyl anion, CH3-
C) methane, CH4
D) ammonia, NH3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following shows curved arrows that correctly accounts for the differences between the two structures? 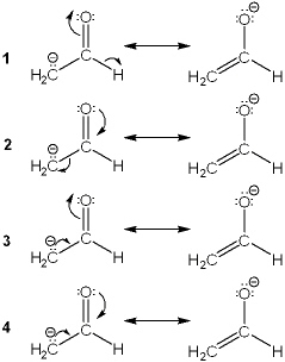
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 95
Related Exams