A) 1 > 2 > 3
B) 1 > 3 > 2
C) 2 > 1 > 3
D) 3 > 1 > 2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is/are tetrahedral? 
A) only 1 and 2
B) only 1 and 3
C) only 1 and 4
D) only 2 and 3
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is an alcohol?
A) CH3CH2COOH
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) CH3CH2CHO
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the highest electronegativity?
A) C
B) P
C) Si
D) Cl
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Convert the following structure into a bond-line drawing.
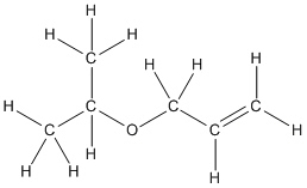
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ammonia (NH3) and which contain the lone pair of electons. Label each orbital with its hybridization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the circled bonds is the strongest? 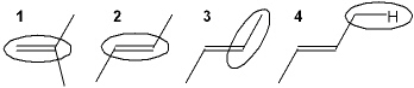
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Circle and name the functional groups in the following molecule.
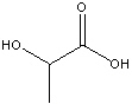
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the primary (1 ) alcohols that have the formula C5H12O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethane, CH3CH3?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the alcohols that have the formula C4H10O.
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the ketones that have the formula C5H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon s and p bonding molecular orbitals of ethene, H2C=CH2?
A) C2sp3 + C2sp3, and C2p + C2p
B) C2sp2 + C2sp2, and C2sp2 + C2sp2
C) C2sp2 + C2sp2, and C2p + C2p
D) C2sp3 + C2sp3, and C2sp2 + C2sp2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H-C-H bond angles in methane, CH4?
A) 90
B) 109
C) 120
D) 180
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the molecular formula of aspartame, shown below?
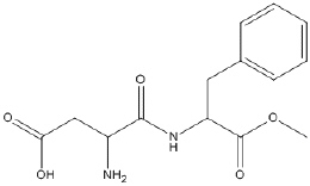
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the aldehydes that have the formula C5H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary amine?
A) CH3CH2N(CH3) 2
B) (CH3) 3CNH2
C) CH3CH2NHCH3
D) CH3CH2NHCH(CH3) 2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the length of the CºC bond in ethyne, HCºCH?
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H-C-H bond angles in a methyl anion, CH3-?
A) 90
B) 109
C) 120
D) 180
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) The sp3C-H bond of an alkane is weaker than the spC-H bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon bond of alkenes.
C) The carbon-carbon triple bond of an alkene is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The sp3C-H bond of an alkane is longer than the spC-H bond of an alkyne.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 95
Related Exams