A) element.
B) isotope.
C) compound.
D) ion.
E) atom.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical bonds involve
A) the giving and taking of electrons.
B) the giving and taking of protons.
C) the giving, taking, or sharing of electrons.
D) the giving, taking, or sharing of protons.
E) the sharing of electrons.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
When a positively charged hydrogen in a water molecule become attracted to thenegatively charged oxygen in a nearby water molecule, this is called a ionic bond.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All atoms of the same element have the same
A) number of neutrons.
B) atomic number.
C) number of electrons.
D) atomic mass.
E) number of ions.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
After drinking a great deal of coffee (pH 5) , a human's blood buffering system would need to ________ as the coffee was digested to lower the level of acid present in the blood stream.
A) release OH -
B) take up H +
C) release H +
D) take up OH -
E) release OH - and take up H +
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following examples, which best demonstrates the property of water cohesion?
A) Water can move up a 100-foot pine tree, from the roots to the leaves.
B) A rock skipping across the surface of a lake.
C) Water requires a great deal of heat to reach the point of vaporizing.
D) A can of soda bursts when it is placed in the freezer.
E) A large body of fresh water takes a long time to warm up after the winter season.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the structure of how water molecules form?
A) Hydrogen atoms covalently bond with each other to create one stable valence shell of electrons. The hydrogen molecule then forms a hydrogen bond with an oxygen atom to create the water molecule.
B) One oxygen atom transfers an electron to each of two hydrogen atoms, forming an ionic bond.
C) The oxygen atom and hydrogen atoms form a covalent bond with one another to create stable valence shells of electrons. The electrons are shared unequally resulting in a polar molecule.
D) Hydrogen bonds are formed between the two hydrogen atoms and the oxygen atom.
E) Because of its strong electronegativity, oxygenremoves one electron from two different hydrogen atoms. This satisfies the valence shell of oxygen. Then hydrogen bonds form between the two hydrogen atoms which keeps them in the vicinity of the oxygen atom.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom that has an electrical charge is called a(n)
A) ion.
B) molecule.
C) isotope.
D) element.
E) proton.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The subatomic particles that are found in the nucleus of an atom are the
A) protons and electrons.
B) neutrons and protons.
C) electrons only.
D) protons only.
E) electrons and neutrons.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure below is depicting the interaction of water molecules with one another. The weak attractions between water molecules are known as 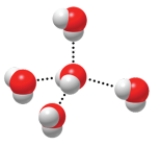
A) covalent bonds.
B) hydrogen bonds.
C) ionic bonds.
D) postive and negative ions.
E) no chemical bonding.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Although Oregon and South Dakota are at similar latitudes, winters in Oregon arewarmer and summers in Oregon are cooler. Which of the following might explain these differences between the climate of Oregon and the climate of South Dakota?
A) South Dakota has fewer trees.
B) The Pacific Ocean makes Oregon temperatures more moderate.
C) Oregon receives more rainfall.
D) South Dakota has fewer lakes and rivers.
E) South Dakota has more prevailing winds from the west.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following properties of water involve hydrogen bonds? Check all that apply
A) cohesion
B) high heat capacity
C) surface tension
D) polarity
E) water expands as it freezes
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which property of water causes sugar to dissolve in coffee?
A) Water has high heat capacity.
B) Water is less dense than ice.
C) Water is a good solvent.
D) Water is cohesive.
E) Water is able to change from a gas to a solid.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a property of water?
A) water is a good solvent
B) water is cohesive
C) water has a high heat capacity
D) water can be found as a solid, liquid, or gas
E) All of the above are properties of water.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be considered a trace element in living things?
A) oxygen
B) carbon
C) hydrogen
D) nitrogen
E) zinc
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The type of bond that would form from the transfer of an electron from one atom to another is a(n) ________ bond.
A) covalent
B) ionic
C) hydrogen
D) atomic
E) isotopic
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic structure of water satisfies the octet rule by having
A) electrons shared between the two oxygen atoms.
B) electrons from hydrogen transferred to the oxygen atom.
C) electrons from oxygen transferred to the hydrogen atoms.
D) oxygen share electrons with two hydrogen atoms.
E) electrons shared between the two hydrogen atoms.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom's valence electron shell
A) is filled when it has three electrons.
B) determines its chemical reactivity.
C) determines its atomic mass.
D) is filled with positively charged particles.
E) is filled identically for every element.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the chemical equation: 6 CO 2+ 6 H 2O + light → C 6H 12O 6+ 6 O 2 , identify which of the following statements are correct. Check all that apply.
A) CO 2 is a reactant
B) H 2O is a product
C) C 6H 12O 6 is both a reactant and a product
D) O 2 is a product.
E) There is not enough information to distinguish the reactants from the products.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of an atom differ in their
A) atomic number.
B) atomic mass.
C) number of electrons.
D) atomic radius.
E) number of protons.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 55
Related Exams