A) reactants.
B) products.
C) enzymes.
D) elements.
E) ions.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When water boils
A) hydrogen bonds are broken between neighboring water molecules.
B) covalent bonds are broken between oxygen and hydrogen atoms.
C) ionic bonds are broken when the minerals in water are heated.
D) the bond between one water molecule and another becomes stronger.
E) the hydrogen atoms break away from the oxygen and escape as vapor.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a way that isotopes are useful in medicine? Check all that apply.
A) Radioactive isotopescan be used as tracers to detect molecular changes or to destroy abnormal or infectious cells.
B) Radioactive isotopes can be used to regenerate damaged tissues.
C) Radioactive isotopes can be used to detect Alzheimer's disease.
D) Radioactive isotopes can be used to sterilize medical equipment.
E) Radioactive isotopes can be used to protect against dangerous biological agents.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of an element differ in their
A) proton number.
B) electron number.
C) neutron number.
D) type of bonds.
E) atomic number.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur has an atomic number of 16. How many valence shell electrons does it have?
A) One
B) Two
C) Three
D) Four
E) Six
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following property of water helps an individual who is exercising vigorously maintain a constant body temperature?
A) Water has high heat capacity.
B) Water is less dense than ice.
C) Water is a good solvent.
D) Water molecules are cohesive.
E) Water moleculesare adhesive.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cola has a pH of 3.5. This means that it has an excess of _______ ions and would be called a(n) ________.
A) H +; acid
B) OH -; acid
C) H +; base
D) OH -; base
E) H +; neutral solution
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about basic solutions is true?
A) Basic solutions have high concentrations of H +.
B) Basic solutions have a pH under 7.
C) Basic solutions release OH -.
D) Basic solutions release both H+ and OH -.
E) Basic solutions have a neutral pH.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some insects can stride on the surface of water because water
A) has a high specific heat.
B) has lower density when frozen.
C) is a good solvent.
D) has surface tension.
E) resists temperature changes.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ionic bond forms when
A) an atom gives away or takes in an electron.
B) an atom gives away or takes in a proton.
C) a negatively charged ion is attracted to one with a positive charge.
D) two atoms come close enough to share one or more electrons.
E) two atoms come close enough to share one or more protons.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a molecule?
A) H 2O
B) O 2
C) NaCl
D) CO 2
E) MgCl 2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom with a neutral charge has
A) equal numbers of neutrons and electrons.
B) more neutrons making it more neutral.
C) the same number of protons and neutrons.
D) equal numbers of protons and electrons.
E) more protons than it does electrons.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cells need buffering agents in order to
A) speed up chemical reactions.
B) carry out life functions in extremely acidic conditions.
C) minimize the changes in pH of their internal environment.
D) help transfer electrons from one atom to another.
E) increase the amount of OH - in their surroundings.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A water molecule, as shown here, is polar because of 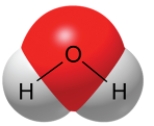
A) the transfer of electrons.
B) unequal sharing of electrons.
C) its ability to freeze.
D) its hydrogen bonds.
E) the negative charge of the molecule.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Heartburn occurs when stomach acid escapes the stomach and burns the tissues of the esophagus. Baking soda is sometimes used as an antacid. How does baking soda help relieve symptoms of heartburn?
A) The baking soda is serving as a buffer to take up excess H + ions from stomach acid.
B) The baking soda is able to coat the lining of the esophagus thereby protecting it from the acid.
C) The baking soda increases the acidity of the stomach.
D) The baking soda releases salt which draws more water into the esophagus thereby diluting the acid.
E) The baking soda relaxes the stomach muscles.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 55 of 55
Related Exams