A) 1% solution.
B) 1 molar solution.
C) 10% solution.
D) 1 osmolal solution.
E) None of these choices are correct.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
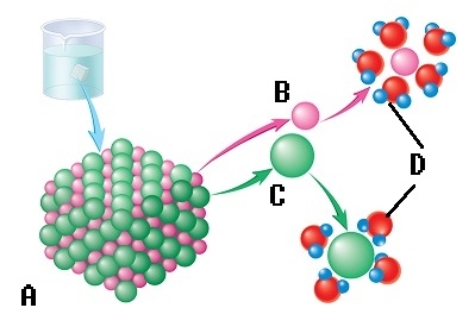 -The sodium chloride molecule breaks apart in water. What does "D" represent?
-The sodium chloride molecule breaks apart in water. What does "D" represent?
A) chloride ion
B) dissociation
C) water molecule
D) sodium ion
E) salt crystal
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Enzymes are proteins that increase the rate of chemical reactions by
A) increasing the activation energy of the reaction.
B) increasing the concentration of the reactants.
C) decreasing the activation energy of the reaction.
D) adjusting the temperature of the reaction.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom has an atomic number of 19 and a mass number of 39. This atom will have
A) 19 neutrons.
B) 39 neutrons.
C) 20 electrons.
D) 20 neutrons.
E) 58 neutrons.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Large carbohydrates are formed from smaller units called
A) lipids.
B) phosphate groups.
C) amino acids.
D) monosaccharides.
E) steroids.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the building block molecule of a protein?
A) monosaccharide
B) amino acid
C) nucleic acid
D) fatty acid
E) glycerol
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is determined by sequence of amino acids bound by peptide bonds?
A) primary structure of protein
B) secondary structure of protein
C) amino acid
D) denaturation
E) peptide bond
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen bonds form between molecules containing _________ bonds; the hydrogen bond is between a hydrogen atom of one molecule and a partially _________ charged atom of another.
A) polar covalent; negatively
B) nonpolar covalent; negatively
C) nonpolar covalent; positively
D) polar covalent; positively
E) ionic; positively
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 161 - 168 of 168
Related Exams