A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 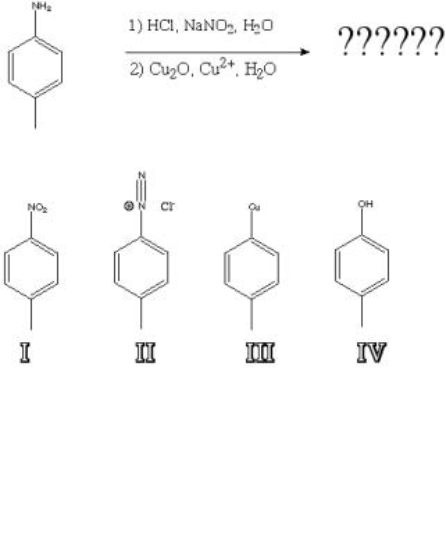
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the structure of N,N-diethylpentanamine? 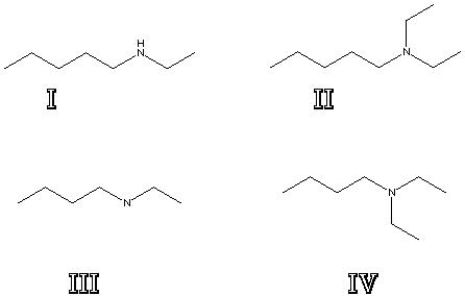
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 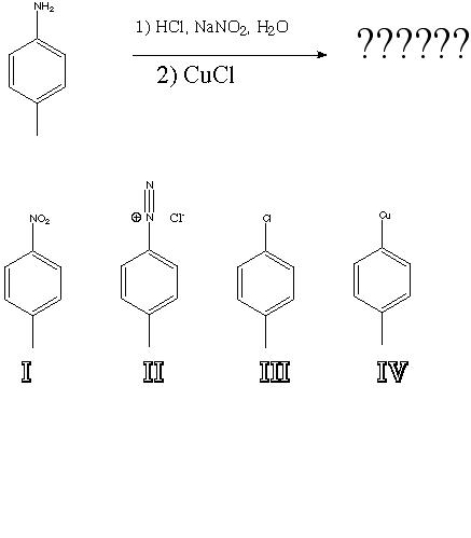
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 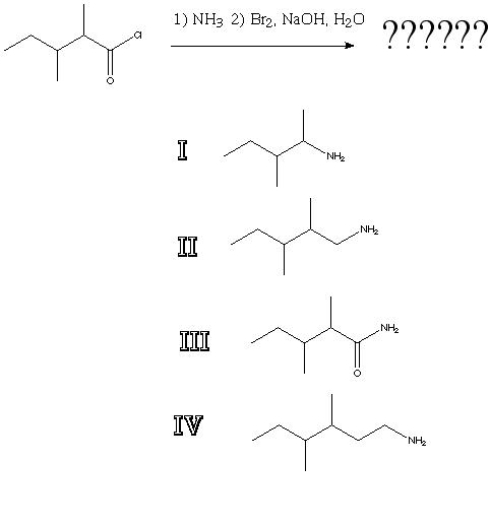
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 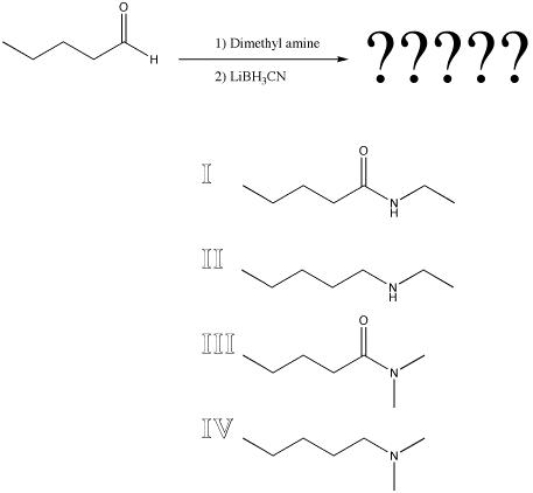
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does pyrrole have a lower pKa (weaker base) than pyridine or pyrimidine? 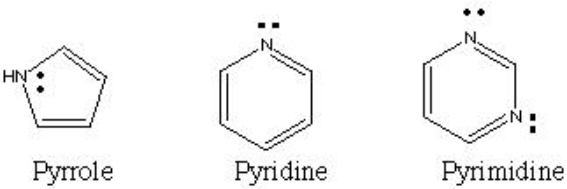
A) Because pyrrole is smaller and more compact and this prevents its lone pair from reacting as much.
B) Because pyrrole has less carbons and therefore the lone pair is more active.
C) Because pyrrole's lone pair participates in the delocalized electron cloud, while pyridine's and pyrimidine's lone pairs do not.
D) Because pyrimidine has more lone pairs than pyrrole does.
F) All of the above
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 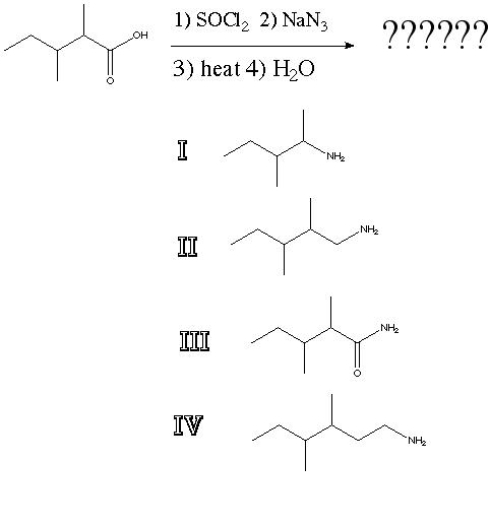
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 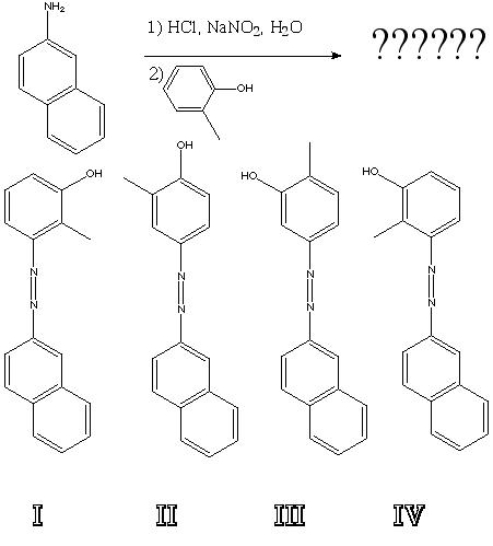
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 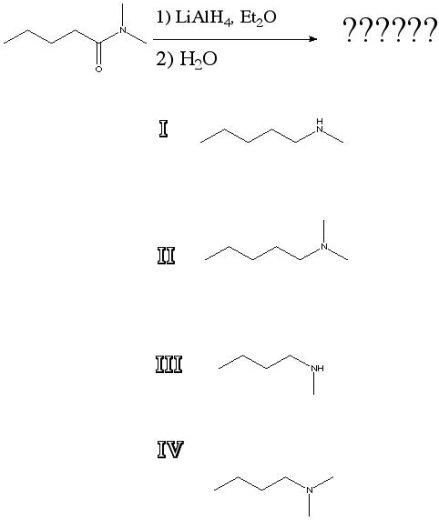
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 10 of 10
Related Exams