A) 0.0186 L
B) 4.5 L
C) 11.2 L
D) 49.2 L
E) 53.7 L
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of hydrogen gas was collected over water at 21 C and 685 mmHg. The volume of the container was 7.80 L. Calculate the mass of H2(g) collected. (Vapor pressure of water = 18.6 mmHg at 21 C.)
A) 0.283 g
B) 0.572 g
C) 0.589 g
D) 7.14 g
E) 435 g
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is consistent with Boyle's Law concerning an ideal gas
A) At constant temperature and moles, a plot of volume versus pressure is linear.
B) At constant pressure and volume, a plot of temperature versus moles is linear.
C) At constant pressure and moles, a plot of temperature versus volume is linear.
D) At constant temperature and moles, a plot of pressure versus the inverse of volume is linear.
E) At constant temperature and pressure, a plot of moles versus volume is linear.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecules of different samples of an ideal gas have the same average kinetic energies, at the same
A) pressure.
B) temperature.
C) volume.
D) density.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a substance that remains a gas under the conditions listed, deviation from the ideal gas law would be most pronounced at
A) 100 C and 2.0 atm.
B) 0 C and 2.0 atm.
C) -100 C and 2.0 atm.
D) -100 C and 4.0 atm.
E) 100 C and 4.0 atm.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
2.0 L of gas A at 1.0 atm and 1.0 L of gas B at 1.0 atm are combined in a 3.0 L flask. The flask is sealed and over time they react completely to give gas C according to the following chemical equation: 2A(g) + B(g) C(g) Assuming the temperature stays constant, what will be the pressure after the reaction goes to completion
A) 0.33 atm
B) 0.50 atm
C) 0.67 atm
D) 0.75 atm
E) 1.0 atm
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of Ar gas are present in a 340 mL container at 55 C and 720 mmHg
A) 0.012 Ar atoms
B) 7.2 x 1021 Ar atoms
C) 4.3 x 1022 Ar atoms
D) 2.9 x 1023 Ar atoms
E) 1.7 x 1024 Ar atoms
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the density, in g/L, of N2 gas at 35 C and 0.98 atm pressure.
A) 0.039 g/L
B) 0.34 g/L
C) 0.54 g/L
D) 1.1 g/L
E) 9.6 g/L
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pressure (mmHg) of the sample of gas trapped in the closed-tube mercury manometer shown below if atmospheric pressure is 751 mmHg and h = 17.3 cm 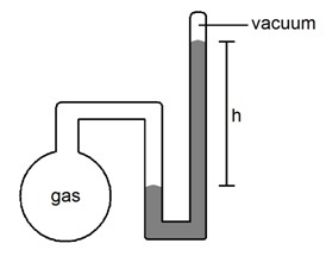
A) 173 mmHg
B) 273 mmHg
C) 373 mmHg
D) 473 mmHg
E) None of the Above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of O2(g) at 810. mmHg pressure is required to react completely with a 4.50g sample of C(s) at 48 C 2 C(s) + O2(g) 2 CO(g)
A) 1.22 L
B) 3.47 L
C) 4.63 L
D) 9.26 L
E) 111 L
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Dalton's Law states that the total pressure of a mixture of gases is just the sum of the pressures that each gas would exert if it were present alone.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mole fraction of oxygen molecules in dry air is 0.2095. What volume of dry air at 1.00 atm and 25 C is required for burning 1.00 L of hexane (C6H14, density = 0.660 g/mL) completely, yielding carbon dioxide and water
A) 187 L
B) 712 L
C) 894 L
D) 1780 L
E) 8490 L
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an effort to address concerns about global warming, a power plant in Portland, Oregon is designed to take all of its exhaust gases from its boilers and recycle the CO2 using the Solvay process to make sodium hydrogen carbonate. The reaction is shown below.NH3(g) + H2O(l) + CO2(g) + NaCl(aq) NaHCO3(aq) + NH4Cl(aq) How many liters each of NH3 and CO2 (both at STP) would be consumed to produce 3.00 kg of sodium bicarbonate
A) The volume of both NH3 and CO2 would be 500. liters.
B) The volume of both NH3 and CO2 would be 600. liters.
C) The volume of both NH3 and CO2 would be 700. liters.
D) The volume of both NH3 and CO2 would be 800. liters.
E) None of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1.000 atm of oxygen gas, placed in a container having a pinhole opening in its side, leaks from the container 2.14 times faster than does 1.000 atm of an unknown gas placed in this same apparatus. Which of these species could be the unknown gas
A) Cl2
B) SF6
C) Kr
D) UF6
E) Xe
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the density, in g/L, of SF6 gas at 27 C and 0.500 atm pressure.
A) 3.38 * 10-3 g/L
B) 2.96 g/L
C) 22.4 g/L
D) 32.9 g/L
E) 3.38 kg/L
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following data describes an initial and final state for an ideal gas. Given that the amount of gas does not change in the process, what is the final pressure (atm) of the gas
A) 1.11 atm
B) 1.21 atm
C) 1.31 atm
D) 1.41 atm
E) None of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant temperature, the volume of the container that a sample of nitrogen gas is in is doubled. As a result the pressure of the nitrogen gas is halved. The amount of nitrogen gas is unchanged in this process. This is an example of:
A) Boyle's Law
B) Avogadro's Law
C) Charles's Law
D) Dalton's Law
E) Gay-Lussac's Law
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Packaged cake mixes usually contain baking powder, a mixture of sodium hydrogen carbonate and calcium hydrogen phosphate that react to produce carbon dioxide gas when they are heated in water. The CO2(g) formed allows the cake to "rise." When such cake mixes are used at high altitudes, often the cake will rise too much and collapse, unless special instructions are followed. Why does this happen
A) Due to the higher atmospheric pressure, a greater volume of carbon dioxide is created.
B) Due to the reduced atmospheric pressure, less volume of carbon dioxide is created.
C) Due to the reduced atmospheric pressure, a greater volume of carbon dioxide is created.
D) Due to the higher atmospheric pressure, less volume of carbon dioxide is created.
E) None of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the volume occupied by 56.5 g of argon gas at STP.
A) 22.4 L
B) 31.7 L
C) 34.6 L
D) 1,270 L
E) 1,380 L
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of pure nitrogen has a temperature of 15 C. What is the temperature of the nitrogen in units of Kelvin
A) 300 K
B) 290 K
C) 288 K
D) 288.2 K
E) 288.15 K
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 130
Related Exams