A) covalent bonds
B) hydrogen bonds
C) ionic bonds
D) valence shells
E) solvents
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a way in which chemical bonds can be formed?
A) sharing electrons
B) losing electrons
C) splitting electrons
D) gaining electrons
E) attractions of opposite charge
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In what ways are radioactive isotopes potentially harmful?
A) Unmonitored release into the environment can make changes in a cell's DNA.
B) They are used to trace molecular changes.
C) They are used to destroy abnormal cells.
D) They are used to determine the age of biological specimens.
E) They are used to trace the path of materials throughout the body.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A covalent bond involves the sharing of
A) electrons.
B) protons.
C) pairs of protons.
D) at least 3 electrons.
E) pairs of electrons.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ionic bond forms when
A) an atom gives away or takes in an electron.
B) an atom gives away or takes in a proton.
C) a negatively charged ion is attracted to one with a positive charge.
D) two atoms come close enough to share one or more electrons.
E) two atoms come close enough to share one or more protons.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the steam being given off when water boils?
A) oxygen molecules
B) hydrogen molecules
C) water molecules
D) hydroxide (OH-) ions
E) hydrogen (H+) ion
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Inside a living cell, which type of bond would be the most stable?
A) hydrogen
B) ionic
C) covalent
D) polar
E) all bonds are equally stable in a living system
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is the smallest unit of matter that has all the properties of an element?
A) molecule
B) element
C) atom
D) compound
E) electron
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The type of bond that would form from the transfer of an electron from one atom to another, as depicted in the figure, is a 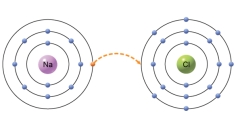
A) covalent.
B) ionic.
C) hydrogen.
D) atomic.
E) isotope.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would not be a valuable use for radioactive isotopes?
A) carbon-14 dating
B) destroying abnormal cells as a type of cancer treatment
C) tracing the path of various chemicals in the body for imaging
D) determining the age of biological specimens
E) damaging DNA of healthy cells.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which property of water would help to account for how an individual who is exercising and producing excessive heat can maintain a constant body temperature?
A) Water has high heat capacity.
B) Water is less dense as ice.
C) Water is a good solvent.
D) Water is cohesive.
E) Water molecules form by covalent bonding.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom that has an electrical charge is called a(n)
A) ion.
B) molecule.
C) isotope.
D) element.
E) proton.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a property of water?
A) It is a good solvent.
B) It is denser when frozen than when liquid.
C) It is cohesive.
D) It resists temperature changes.
E) It can be found as a solid, liquid, or gas.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A covalent bond occurs when ________.
A) protons are transferred from one atom to another
B) neutrons are shared between two atoms to form an isotope
C) electrons are shared between two atoms to complete their octets
D) the hydrogen of one water molecule is attracted to the oxygen of another water molecule
E) electrons are transferred from one atom to another
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do a strong acid and a weak acid differ?
A) A strong acid has less H+ in solution.
B) A weak acid dissociates only partially in water.
C) A strong acid is less likely to remain dissociated.
D) A weak acid dissociates nearly completely in water.
E) A strong acid dissociates only partly in water.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A water molecule, as shown here, is polar because of ________. 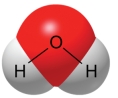
A) transfer of electrons
B) unequal sharing of electrons
C) its ability to freeze
D) its hydrogen bonds
E) its change in density when frozen
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur has an atomic number of 16. What would be the valence number of this element?
A) One
B) Two
C) Three
D) Four
E) Six
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The term to describe any substance which can prevent the pH of a solution from changing by either releasing or absorbing H+ in a solution is
A) equalizer.
B) solute.
C) buffer.
D) acid.
E) base.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describe how an acid disrupts the chemical bonds of molecules in a cell?
A) the H+ ions can disrupt hydrogen bonds as the slightly negative portion of the molecule is more attracted to it than to the hydrogen that was part of the bond.
B) the H+ ions can disrupt hydrogen bonds as the slightly positive portion of the molecule is more attracted to it than to the hydrogen that was part of the bond.
C) the OH- ions can disrupt hydrogen bonds as the slightly positive portion of the molecule is more attracted to it than to the hydrogen that was part of the bond.
D) the OH- ions can disrupt hydrogen bonds as the slightly negative portion of the molecule is more attracted to it than to the hydrogen that was part of the bond.
E) The H+ ions disrupt the covalent bonds that hold the molecule together.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a property of acids?
A) release hydrogen ions when dissolved in a liquid
B) feel slippery when touched
C) taste bitter
D) release hydroxide ions when dissolved in a liquid
E) have a pH reading above 7.0
G) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 62
Related Exams