A) Br-Br
B) C-Cl
C) C-S
D) Na-O
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the circled bonds is the strongest? 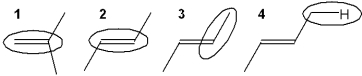
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 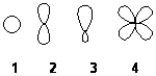
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is a polar covalent bond?
A) Na-F
B) C-H
C) C-O
D) Cl-Cl
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is a polar covalent bond?
A) Na-Cl
B) C-Cl
C) C-H
D) Cl-Cl
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the tertiary (3 ) amines that have the formula C5H11N.
Correct Answer

verified
11ea7d75_f5da_bd13_b9bd_136ab4437de0_TB1813_00_TB1813_00
Correct Answer
verified
Essay
Convert the following structure into a bond-line drawing. 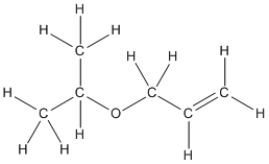
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment? 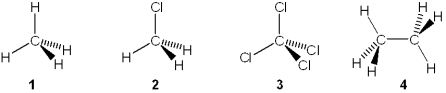
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the alkanes that have the formula C5H12.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a primary (1 ) amine? 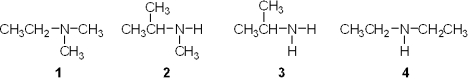
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
C
Correct Answer
verified
Essay
Convert the following structure into a bond-line drawing. 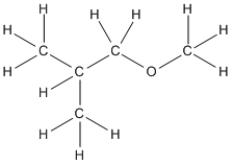
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the molecular formula of Ritalin, shown below? 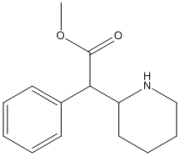
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H-C-H bond angles in a methyl cation, CH3+?
A) 90
B) 109
C) 120
D) 180
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
What is the molecular formula of aspartame, shown below? 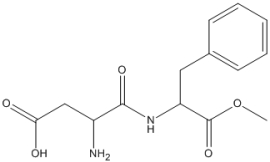
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) The sp3C-H bond of an alkane is weaker than the spC-H bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon bond of alkenes.
C) The carbon-carbon triple bond of an alkene is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The sp3C-H bond of an alkane is longer than the spC-H bond of an alkyne.
F) All of the above
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular bromine, Br2
B) fluoride anion, F-
C) ammonia, NH3
D) aluminum trichloride, AlCl3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment?
A) H2O
B) CO2
C) HC CH
D) Cl2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Overlap of the two atomic orbitals as shown could result in the formation of a bond. 

B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has an atom that has an unfilled valence shell of electrons?
A) molecular hydrogen, H2
B) hydroxide anion, HO-
C) boron trifluoride, BF3
D) water, H2O
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following resonance structures makes the largest contribution to the structure of [H2CCHO]-? ![Which of the following resonance structures makes the largest contribution to the structure of [H<sub>2</sub>CCHO]<sup>-</sup>? A) 1 B) 2 C) 3 D) 4](https://d2lvgg3v3hfg70.cloudfront.net/TB1813/11ea7d75_f5d8_c126_b9bd_8bc12cf070dc_TB1813_00_TB1813_00.jpg)
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 115
Related Exams