A) the rate remains the same
B) the rate decreases by a factor of 2
C) the rate increases by a factor of 2
D) the rate increases by a factor of 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 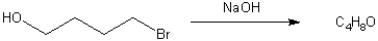
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 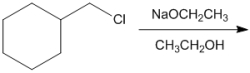
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For each substrate below, choose which reaction type is favored. Place the letter of the reaction type in the blank to the left of the substrate.
-______ 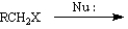
A) SN1
B) SN2
C) E1
D) E2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol leading to elimination? 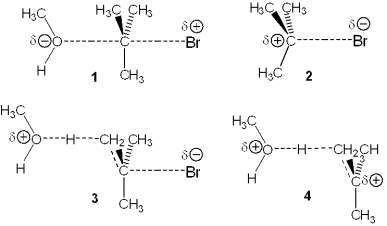
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The first step in the mechanism for the following reaction would be the formation of a secondary carbocation. 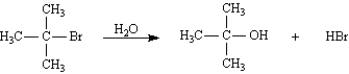
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major elimination product obtained from the following reaction? 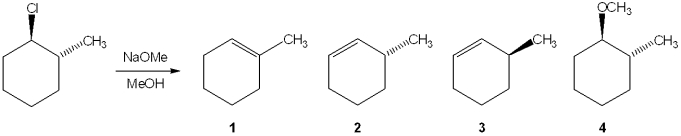
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of 1-bromopropane with sodium iodide gives 1-iodopropane. What is the effect of doubling the concentration of NaI on the rate of the reaction?
A) the rate remains the same
B) the rate decreases by a factor of 2
C) the rate increases by a factor of 2
D) the rate increases by a factor of 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 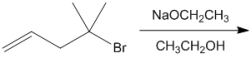
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn mechanism for the following reaction, including curved arrows to show the movement of pairs of electrons and the structure of reactive intermediates. 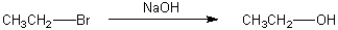
Correct Answer

verified
The reaction proceeds in a sin...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following alkyl bromides reacts the slowest with NaSCH3 in DMF? 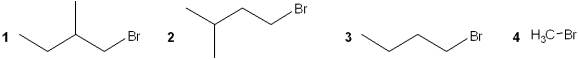
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify each of the following species ,Place the letter of the choice in the blank to the left of the formula.
-________ 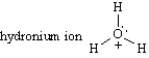
A) an electrophile.
B) a nucleophile.
C) either an electrophile or a nucleophile.
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams represents the course of an exothermic E1 reaction? 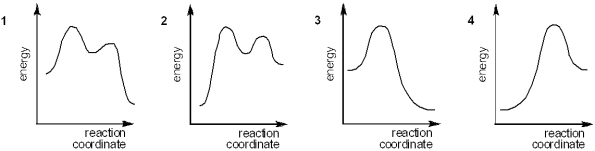
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the transition state of the rate-determining step in the reaction between 2-bromopropane and sodium methoxide leading to elimination? 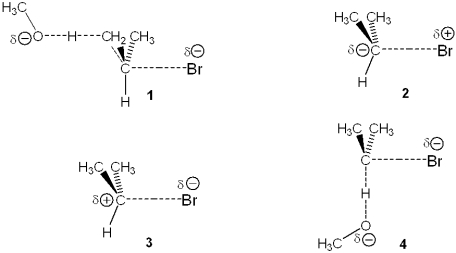
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the transition state of the reaction between methyl bromide and sodium methylthiolate, NaSCH3? 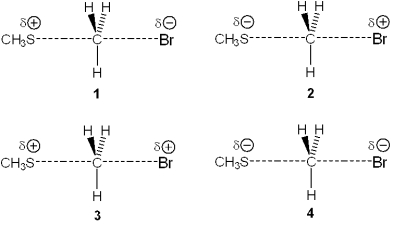
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
In the following reaction the cyanide ion is both a nucleophile and a Lewis acid. 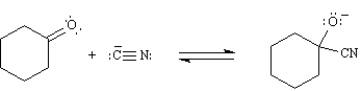
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions corresponds to a substitution?
A) propene 1,2-dibromopropane
B) 1-iodopropane propene
C) propene propane
D) 1-iodopropane 1-bromopropane
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The rate law for the following reaction would be of the form Rate = k[A][B]. ![The rate law for the following reaction would be of the form Rate = k[A][B].](https://d2lvgg3v3hfg70.cloudfront.net/TB1813/11ea7d75_f5fc_00ae_b9bd_63b901bf68bf_TB1813_00_TB1813_00.jpg)
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to perform the following transformation? 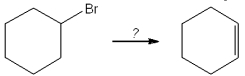
A) H2SO4
B) H2O
C) NaOCH3
D) KOtBu
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams represents the course of an exothermic SN1 reaction? 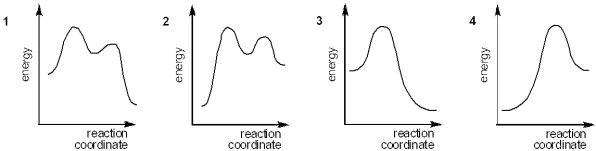
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 111
Related Exams