A) no bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) ionic bonds
E) covalent bonds
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms with greatly different electronegativity values are expected to form
A) no bonds
B) covalent bonds
C) triple bonds
D) ionic bonds
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a valid resonance structure for N3-?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) all are correct
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the elements Cs,F,and P,the order of increasing electronegativity is:
A) Cs < F < P
B) Cs < P < F
C) P < F < Cs
D) F < Cs < P
E) none of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the electron dot formula that most accurately describes the bonding in CS2.(Hint: Consider formal charges. )
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for the given species from the choices below: -PF5
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular structure of AsCl5 is
A) trigonal bipyramidal
B) square pyramidal
C) distorted tetrahedral
D) octahedral
E) none of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for the given species from the choices below: -BeCl2
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) none of these
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following Lewis structure: 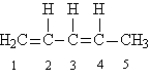 -How many unshared pairs of electrons are present in this molecule?
-How many unshared pairs of electrons are present in this molecule?
A) 0
B) 1
C) 2
D) 3
E) 4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecules. I.BF3 II.CHBr3 (C is the central atom) III.Br2 IV.XeCl2 V.CO VI.SF4 Select the molecule(s) that fit the given statement. -These molecules have a trigonal bipyramidal electron pair arrangement.
A) II,IV,VI
B) I,IV
C) IV,VI
D) VI only
E) none of them
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains a double bond?
A) CO2
B) NH3
C) H2O
D) all
E) none
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structures of the molecules below and use them to answer the following questions: I.BH3 II.NO2 III.SF6 IV.O3 V.PCl5 -Which of these molecules show resonance?
A) I,II
B) II,IV
C) II,V
D) III,IV
E) III,V
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct order for molecules from most to least polar?
A) CH4 > CF2Cl2 > CF2H2 > CCl4 > CCl2H2
B) CH4 > CF2H2 > CF2Cl2 > CCl4 > CCl2H2
C) CF2Cl2 > CF2H2 > CCl2H2 > CH4 = CCl4
D) CF2H2 > CCl2H2 > CF2Cl2 > CH4 = CCl4
E) CF2Cl2 > CF2H2 > CCl4 > CCl2H2 > CH4
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which pair do both compounds exhibit predominantly ionic bonding?
A) SCl6 and HF
B) Na2SO3 and NH3
C) KI and O3
D) LiF and H2O
E) LiBr and MgO
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic compounds has the largest lattice energy (i.e. ,the lattice energy most favorable to a stable lattice) ?
A) BaO
B) BeO
C) CsI
D) NaBr
E) BaS
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for the given species from the choices below: -ClO2
A) pyramidal
B) tetrahedral
C) square planar
D) octahedral
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of molecules always has a dipole moment?
A) Linear molecules with two identical bonds.
B) Tetrahedral molecules (four identical bonds equally spaced) .
C) Trigonal pyramid molecules (three identical bonds) .
D) Trigonal planar molecules (three identical bonds equally spaced) .
E) None has a dipole moment.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
For each of the following compounds: a)Draw the Lewis structure. b)Give the shape of the molecule. c)Indicate the polarity of the molecule. -NH3
Correct Answer

verified
Part A:  P...
P...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
In the Lewis structure for elemental nitrogen there is (are)
A) a single bond between the nitrogens
B) a double bond between the nitrogens
C) a triple bond between the nitrogens
D) three unpaired electrons
E) none of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is best described by drawing resonance structures?
A) PH3
B) NH4+
C) O3
D) SO3
E) HCN
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 146
Related Exams