A) covalent bonds.
B) hydrogen bonds.
C) ionic bonds.
D) postive and negative ions.
E) no chemical bonding.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would not be a valuable use for radioactive isotopes?
A) carbon-14 dating
B) destroying abnormal cells as a type of cancer treatment
C) tracing the path of various chemicals in the body for imaging
D) determining the age of biological specimens
E) damaging DNA of healthy cells
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic structure of water satisfies the octet rule by having
A) electrons shared between the two oxygen atoms.
B) electrons from hydrogen transferred to the oxygen atom.
C) electrons from oxygen transferred to the hydrogen atoms.
D) oxygen share electrons with two hydrogen atoms.
E) electrons shared between the two hydrogen atoms.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Although Oregon and South Dakota are at similar latitudes, winters in Oregon are warmer and summers in Oregon are cooler. Which of the following might explain these differences between the climate of Oregon and the climate of South Dakota?
A) South Dakota has fewer trees.
B) The Pacific Ocean makes Oregon temperatures more moderate.
C) Oregon receives more rainfall.
D) South Dakota has fewer lakes and rivers.
E) South Dakota has more prevailing winds from the west.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The type of bond that would form from the transfer of an electron from one atom to another, shown below, is an) ________ bond. 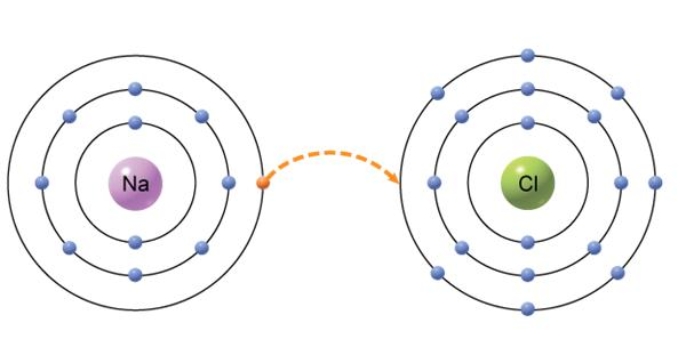
A) covalent
B) ionic
C) hydrogen
D) atomic
E) isotopic
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A covalent bond involves the sharing of
A) electrons.
B) protons.
C) pairs of protons.
D) at least three electrons.
E) pairs of electrons.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following explains the events occurring when water boils?
A) Hydrogen bonds are broken between neighboring water molecules.
B) Covalent bonds are broken between oxygen and hydrogen atoms.
C) Ionic bonds are broken when the minerals in water are heated.
D) The bond between one water molecule and another is strengthened.
E) The hydrogen atoms break away from the oxygen and escape as vapor.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ionic bond forms when
A) an atom gives away or takes in an electron.
B) an atom gives away or takes in a proton.
C) a negatively charged ion is attracted to one with a positive charge.
D) two atoms come close enough to share one or more electrons.
E) two atoms come close enough to share one or more protons.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the structure of how water molecules form and interact?
A) Hydrogen atoms bond with each other to create a stable outer shell of electrons. Then they form a hydrogen bond to an oxygen atom to create the water molecule.
B) Oxygen atoms transfer one electron to each of the hydrogen atoms, forming an ionic bond that attracts other water molecules to it.
C) Oxygen atoms transfer one electron to each of the hydrogen atoms, forming an ionic bond that attracts other water molecules to it
D) Hydrogen bonds are formed between the two hydrogen atoms and the oxygen atom. This water molecule then forms a covalent bond with adjacent water molecules.
E) The oxygen atom is more electronegative than the two hydrogen atoms. Due to this, it removes the electron from each hydrogen atom. This satisfies the outer shell of oxygen. Then hydrogen bonds form between the two remaining hydrogen atoms to hold them near to the oxygen atom.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Baking soda is sometimes used as an antacid. The chemical name for baking soda is sodium bicarbonate. What is the bicarbonate doing to help with stomach upset?
A) It is serving as a buffer to take up excess H+ ions from stomach acid.
B) It is able to coat the stomach lining.
C) The bicarbonate helps to create more acid in the stomach.
D) The bicarbonate acts as a strong acid quickly dissociating into H+ ions.
E) It relaxes the stomach muscles.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following examples, which best demonstrates the property of water cohesion?
A) Water can move up a 100-foot pine tree, from the roots to the leaves.
B) A rock skipping across the surface of a lake.
C) Water requires a great deal of heat to reach the point of vaporizing.
D) A can of soda bursts when it is placed in the freezer.
E) A large body of fresh water takes a long time to warm up after the winter season.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of an element differ in their
A) proton number.
B) electron number.
C) neutron number.
D) type of bonds.
E) atomic number.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the steam being given off when water boils?
A) oxygen molecules
B) hydrogen molecules
C) water molecules
D) hydroxide OH-) ions
E) hydrogen H+) ions
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction 6CO2 + 6H2OC6H12O6 + 6O2 carbon dioxide is one of the
A) reactants.
B) products.
C) enzymes.
D) elements.
E) catalysts.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these does not occur when a sodium atom transfers an electron to a chlorine atom?
A) The sodium atom becomes a positively charged ion.
B) The positive and negative ions will attract each other, forming a crystal if no water is present.
C) The ions will separate in the presence of water.
D) There is a mutual sharing of the electrons between the sodium and chlorine atoms.
E) The chlorine atom becomes a negatively charged ion.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An) ________ can be defined as a substance that prevents the pH of a solution from changing by either releasing or absorbing H+ in a solution.
A) equalizer
B) solute
C) buffer
D) acid
E) base
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of an atom differ in their
A) atomic number.
B) atomic mass.
C) number of electrons.
D) atomic radius.
E) number of protons.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron arrangement for argon, which has 18 electrons, is
A) 2 in the inner energy shell, 8 in the second energy shell, and 8 in the outer energy shell.
B) 8 in the inner energy shell, 8 in the second energy shell, and 2 in the outer energy shell.
C) 6 in the inner energy shell, 6 in the second energy shell, and 6 in the outer energy shell.
D) 5 in the inner energy shell, 6 in the second energy shell, and 7 in the outer energy shell.
E) 7 in the inner energy shell, 6 in the second energy shell, and 5 in the outer energy shell.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does a strong acid differ from a weak acid?
A) A strong acid contains fewer H+ in solution.
B) A weak acid dissociates only partially in water.
C) A strong acid is less likely to remain dissociated.
D) A weak acid dissociates nearly completely in water.
E) A strong acid dissociates only partly in water.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be an example of the value of water's heat capacity?
A) Water is able to travel up a 100-foot tree.
B) Water expands as it freezes causing ice to float on the surface of a lake.
C) Living organisms are able to maintain their internal body temperatures because the water in their cells resists changes in temperature.
D) Small insects can walk on water.
E) Ice cubes float.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 55
Related Exams