A) Zeroth
B) First
C) Second
D) Third
E) The rate constant cannot be determined using only a rate constant for any reaction.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following potential energy profile for the A → B reaction.The overall reaction is________. 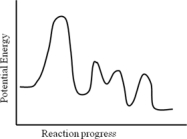
A) endothermic
B) exothermic
D) undefined
Correct Answer

verified
Correct Answer
verified
Essay
Is a bimolecular reaction necessarily second-order? Is a second order reaction necessarily bimolecular? Answer,with explanations and clarifications.
Correct Answer

verified
For elementary reactions,the order follo...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
For the reaction A + 2B → C,which expression is correct?
A) Δ[C]/Δt = Δ[A]/Δt
B) Δ[C]/Δt = 2Δ[B]/Δt
C) Δ[C]/Δt = -2Δ[B]/Δt
D) Δ[C]/Δt = -½Δ[B]/Δt
E) Δ[C]/Δt = -½Δ[A]/Δt
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The units of the rate constant depend on the order of the reaction.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are possible units for the reaction rate?
A) L • mol-1 • s-1
B) L2 • mol-2 • s-1
C) s-1
D) s-2
E) mol • L-1 • s-1
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
According to the collision theory of reaction rates,what are the three requirements which must be met before an elementary reaction between two molecules can occur?
Correct Answer

verified
Molecules must collide with ea...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
A certain first-order reaction A → B is 25% complete in 42 min at 25°C.What is the half-life of the reaction?
A) 21 min
B) 42 min
C) 84 min
D) 120 min
E) 101 min
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following potential energy profile for the A → B reaction.How many intermediates are formed? 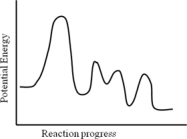
A) 1
B) 2
C) 3
D) 4
E) 5
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-14 is a radioactive isotope which decays with a half-life of 5730 years.What is the first-order rate constant for its decay?
A) 5.25 × 10-5 yr-1
B) 1.21 × 10-4 yr-1
C) 1.75 × 10-4 yr-1
D) 3.49 × 10-4 yr-1
E) 3.97 × 103 yr-1
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Butadiene,C4H6 (used to make synthetic rubber and latex paints) .dimerizes to C8H12 with a rate law of rate = 0.014 L/mol• s [C4H6]2.What will be the concentration of C4H6 after 3.0 hours if the initial concentration is 0.025 M?
A) 0.0052 M
B) 0.024 M
C) 43 M
D) 190 M
E) 0.0000 M
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The rate law predicted by the following two-step mechanism is Rate = k[A][B]. A → C + B (slow) A + B → C + E (fast)
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a zeroth-order reaction,if the concentration of reactant A is plotted vs.time,which corresponds to the slope of this plot?
A) 1/[A]
B) k
C) 1/k
D) ln[A]
E) -k
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the name given to the equation that relates the rate of reaction to the concentrations of reactants?
Correct Answer

verified
Correct Answer
verified
True/False
A transition state is a species (or state)corresponding to an energy maximum on a reaction energy diagram.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Enzymes are _______ .
A) large carbohydrate molecules
B) also called substrates
C) usually heterogeneous catalysts
D) insensitive to temperature
E) biological catalysts
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2NO2(g) → 2NO(g) + O2(g) is suspected to be second order in NO2.Which of the following kinetic plots would be the most useful to confirm whether or not the reaction is second order?
A) A plot of [NO2]-1 vs.t
B) A plot of ln [NO2] vs.t
C) A plot of [NO2] vs.t
D) A plot of ln [NO2]-1 vs.t
E) A plot of [NO2]2 vs.t
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate law for the reaction 3A → 2B is rate = k[A] with a rate constant of 0.0447 h-1.What is the half-life of the reaction?
A) 0.0224 h
B) 0.0645 h
C) 15.5 h
D) 22.4 h
E) 44.7 h
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
According to the ___________ ________ of chemical kinetics,the reaction rate is directly proportional to the number of molecular collisions per second.
Correct Answer

verified
Correct Answer
verified
True/False
The rate of a reaction is determined by the rate of the fastest step in the mechanism.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 114
Related Exams